In everyday life, the terms heat and temperature are often used interchangeably. In physics, however, they refer to different concepts. To understand heat transfer in roasting, it’s important to understand the difference in meaning between the terms.
Heat refers to thermal energy — specifically, the thermal energy that is transferred from one object to another. In scientific notation, energy is measured in joules (J); other common units of energy are calories, kilowatt-hours (kWh), and British thermal units (Btu), used to indicate the energy content in fuel.
 One Btu is approximately equal to the energy released by burning a match.
One Btu is approximately equal to the energy released by burning a match.
Thermal energy is the energy of the movement of atoms or molecules (a group of atoms bonded together) within a material. In a gas, for example, the individual molecules move around at random, bouncing off each other and the walls of the container. The amount of thermal energy determines how fast they move. In a solid, on the other hand, the atoms vibrate in place. If a material has more thermal energy, then they vibrate more strongly.
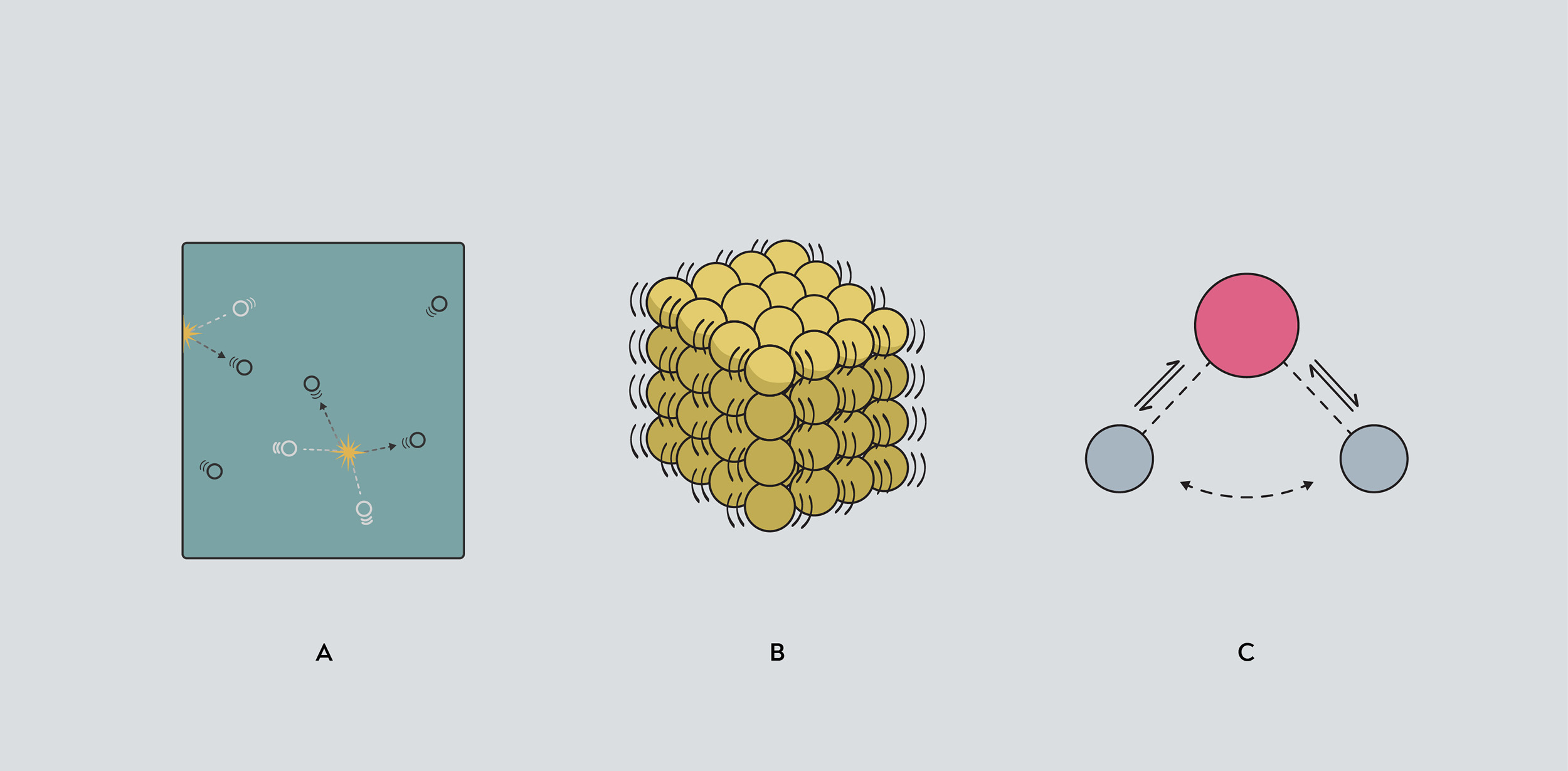 Thermal energy is the energy of atoms moving within a substance, whether that’s individual molecules moving around in a gas (a), atoms vibrating in a solid (b), or atoms shifting their position within a molecule (c).
Thermal energy is the energy of atoms moving within a substance, whether that’s individual molecules moving around in a gas (a), atoms vibrating in a solid (b), or atoms shifting their position within a molecule (c).
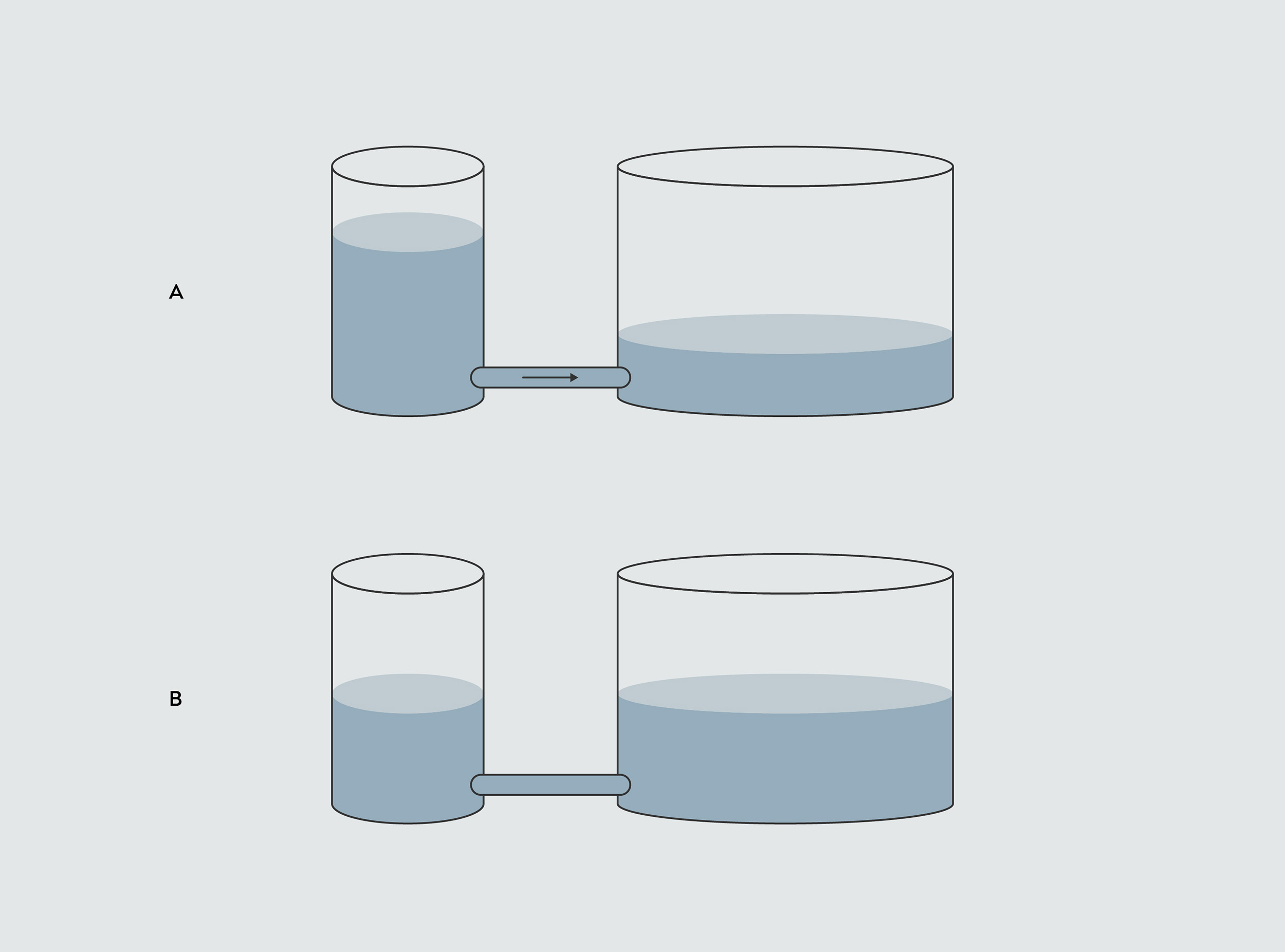
Temperature, on the other hand, is a measure of how ‘willingly’ the substance transfers the thermal energy it contains to another substance. To understand how this works, picture two containers connected by a tube. Each container holds a certain amount of water, representing its thermal energy.
In this analogy, the height of water in each container represents the temperature. Just as water flows from the more full container into the less full one, heat energy flows from a hotter substance to a cooler one, until they reach the same temperature. The temperature difference drives the flow of heat energy.
However, although the two substances have reached the same temperature,