While we know that exothermic reactions take place during coffee roasting, it’s not certain how strongly those reactions affect the bean temperature.
Direct measurements show that the chemical reactions in coffee beans are, on balance, endothermic during the first part of the roast, but they become exothermic at higher temperatures due to the onset of pyrolysis reactions. Estimates of the temperature at which beans become exothermic vary from 140°C up to around 190°C (Schenker 2000).
Measuring the exact amount of heat released by the chemical reactions that take place during roasting is a complex undertaking. The standard method is to gradually increase the temperature of the coffee in a sealed container and measure the amount of heat required to change the temperature at each stage.
However, this is quite different to the conditions inside a coffee roaster, where the beans are heated much more quickly and water and other gases can freely escape from them.
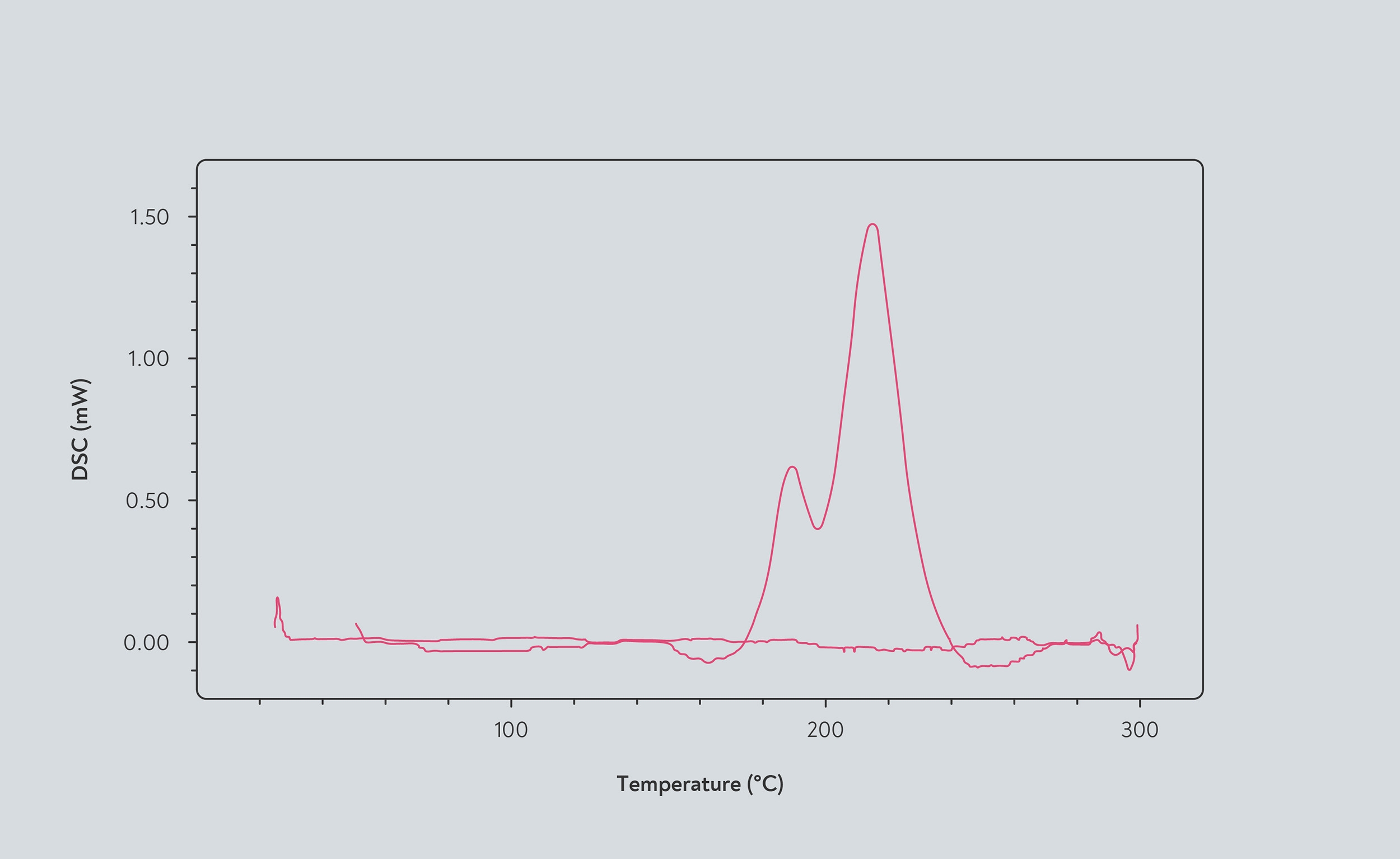 Exothermic reactions in coffee beans shown by differential scanning calorimetry. The red line represents the amount of heat released by chemical processes as the temperature increases. When the line falls below the reference point, the beans are endothermic. When the line rises above the reference point, the beans are exothermic. According to this measurement, the beans become exothermic at 165°C, and the exothermic reactions peak at 210°C. Source: Abdul Ghani et al (2015)
Exothermic reactions in coffee beans shown by differential scanning calorimetry. The red line represents the amount of heat released by chemical processes as the temperature increases. When the line falls below the reference point, the beans are endothermic. When the line rises above the reference point, the beans are exothermic. According to this measurement, the beans become exothermic at 165°C, and the exothermic reactions peak at 210°C. Source: Abdul Ghani et al (2015)
In one study, researchers modelled temperature changes within coffee beans during roasting and calculated the effect that exothermic reactions would have. They found that including exothermic reactions in their model resulted in a final temperature less than 5°C higher than if they ignored those reactions (Abdul Ghani et al 2019). This finding suggests that the role of exothermy in determining the shape of the roast curve is relatively minor.
The Plateau
When roasting coffee, many roasters observe a ‘plateau’ in the rate of rise of the bean temperature in the run up to first crack,