In the last few lessons of our علوم التحميص (قيد الترجمة) course, we’ve been exploring the evaporation front that forms inside coffee beans as they roast. In coffee roasting this phenomenon has an almost mythical status. It has even been touted as a possible cause of first crack, but there’s very little direct study on this topic, and a whole lot of conjecture.
In this post, we’ll take a closer look at what we do know about how moisture evaporates during roasting, and explain some of the reasons why evaporation is so important to how the coffee roasts.
When Does the Moisture Evaporate?
Unfortunately, it’s very hard to accurately measure what happens to the moisture in the bean during the roast. There are essentially two approaches: either take out samples of beans at various stages during the roast, and measure the remaining moisture content in the sample; or measure the moisture being given off in the exhaust gases.
Each approach comes with problems: if you are measuring the water content of samples from the roaster, they have to be cooled first, during which time they could continue releasing moisture, or absorb it from the air. To measure the moisture content with a dehydration oven — the most accurate method — the beans may need to be ground, at which point they may release more moisture or other gases that would affect the result (Schenker 2000).
The other approach, measuring the moisture in the exhaust stack, is technically pretty challenging. It also makes it hard to distinguish between existing moisture in the bean being evaporated, and extra moisture that is created in chemical reactions during roasting.
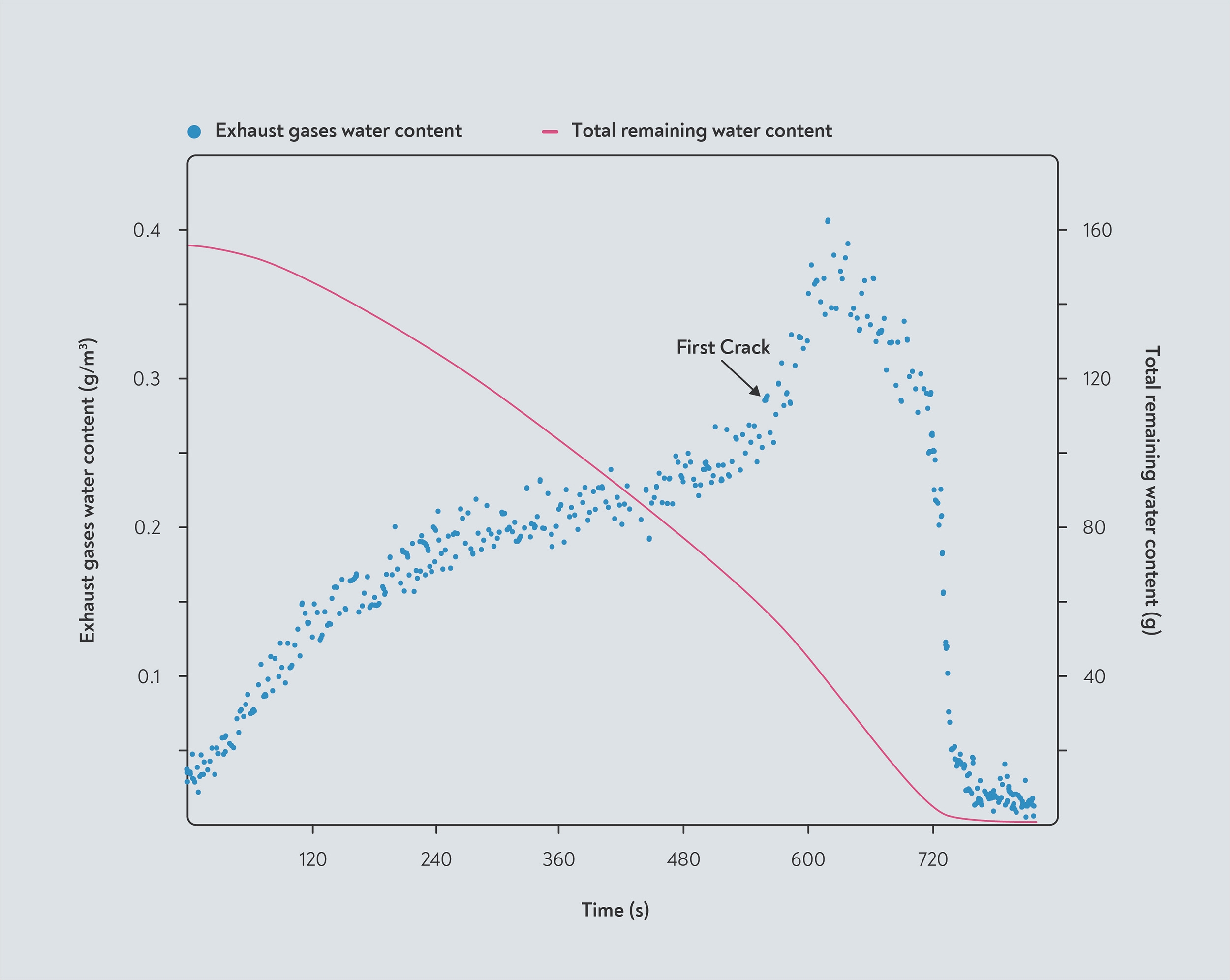
James Davison, a chemical engineer and founder of Williamstown Roasters, took the second approach to measure the moisture given off by beans during roasting. In his results, shown in the graph below right, the total amount of water released makes up about 16% of the initial weight of green beans — but a substantial amount of the water that he detected would have been created during the roast.
 The custom-made anemometer used to measure airflow in the exhaust stack for James Davison’s experiments
The custom-made anemometer used to measure airflow in the exhaust stack for James Davison’s experiments
All of this means that it’s hard to be certain about the moisture content in the bean during roasting, and attempts to measure it often give conflicting results. However, most available evidence suggests that moisture is continually evaporating the whole way through the roast, rather than just during the so-called ‘drying phase’ at the beginning of the roast.
Two approaches to measuring moisture loss. The left-hand graph, adapted from Schenker (2000), shows the moisture content of bean samples removed at different stages of the roast in a fluid-bed roaster operated at a fixed temperature. The right-hand graph, from Davison (2019) is based on the moisture content measured in the exhaust gases during roasting. These measurements were taken in a drum roaster, and show a distinct spike in moisture evaporation at first crack. The total water content is calculated from the moisture evaporated in the stack and therefore includes water created in chemical reactions during roasting.
But this doesn’t mean that the moisture content is decreasing evenly throughout the bean. Instead, for most of the roast, there’s a clear separation between a dry region on the outside of the bean, and a moist region on the inside. Between the two is a layer where moisture is evaporating and escaping from the bean — the evaporation front.
The Evaporation Front
The core idea of the evaporation front is that water has to escape from the outside layer of the bean first. At the beginning of the roast, the bean temperature increases fastest near the bean surface, and heat travels more slowly to the inner layers. The moisture near the bean surface therefore evaporates first, creating a layer of water vapour in the outer part of the bean — the evaporation front.
As the bean temperature rises past 100°C, the pressure inside the bean begins to rise as steam builds up faster than it can escape. The pressure can reach as high as 25 bars during the roast (Bonnländer et al 2005) — more than twice the amount of pressure you’ll find in an Olympic track bike tyre. The high pressure makes it harder for water to evaporate, so the bean still contains liquid water, or water bound to the bean structure, at well over boiling point. This works in just the same way that the steam boiler in your espresso machine contains both steam and water at 120°C or more: the pressure prevents water from evaporating, which allows the temperature to increase past the usual boiling temperature.
In the outer layers of the bean, the steam can escape through the bean’s pores, the pressure drops, and the remaining water evaporates. In the inner layers of the bean, the steam can’t escape, because it is surrounded by high pressure steam on all sides.
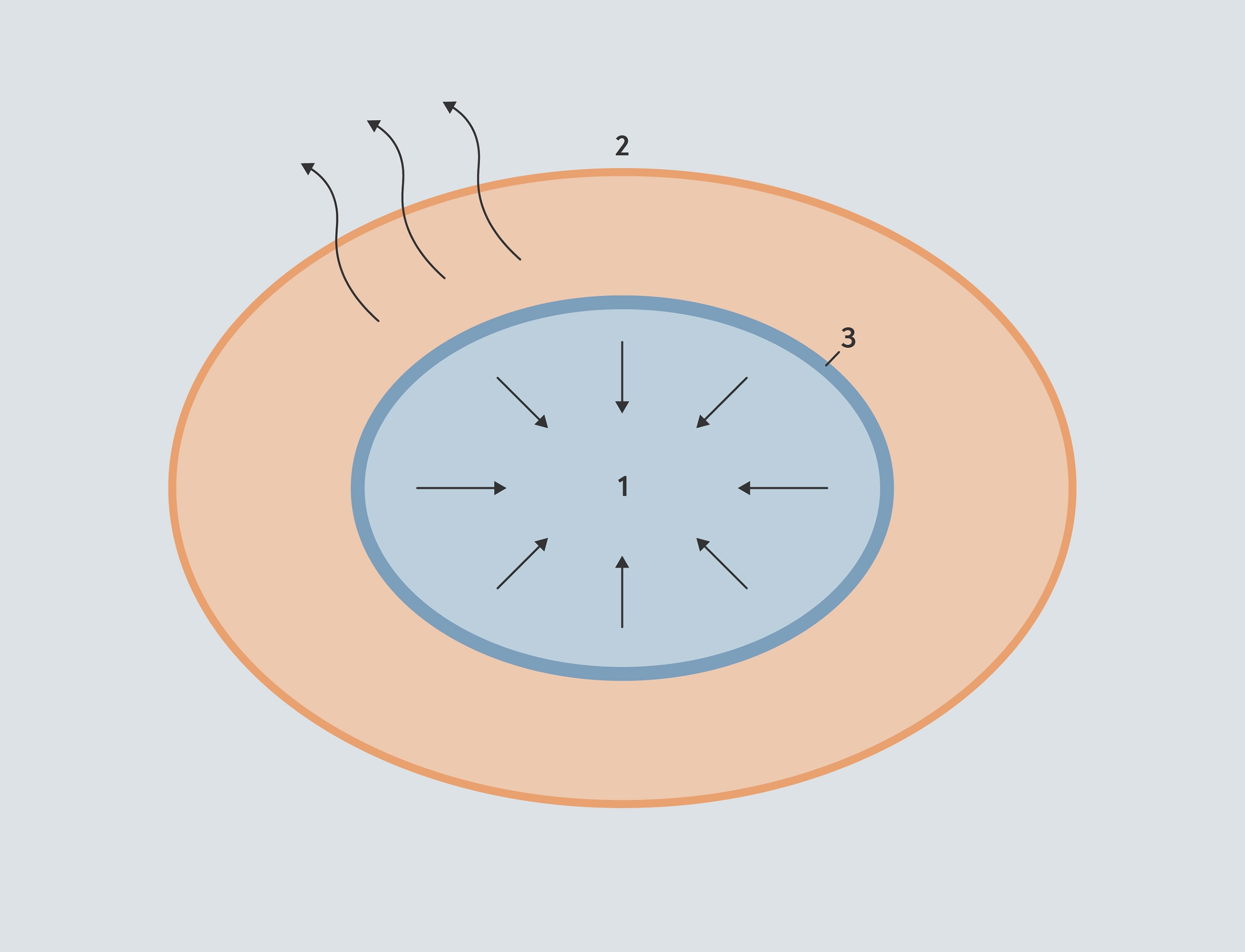 The evaporation front. The core of the bean (1) contains both liquid/bound water and steam at high pressure. The steam can’t escape, because it is surrounded by high pressure on all sides. Steam escapes from the outer layer of the bean (2). In between the two is the layer where the liquid/bound water is able to turn to steam — the evaporation front (3).
The evaporation front. The core of the bean (1) contains both liquid/bound water and steam at high pressure. The steam can’t escape, because it is surrounded by high pressure on all sides. Steam escapes from the outer layer of the bean (2). In between the two is the layer where the liquid/bound water is able to turn to steam — the evaporation front (3).
The result is a dry layer on the outside of the bean, while the centre still contains plenty of moisture. As the roast continues, the evaporation front moves inwards towards the centre of the bean as more and more of the steam escapes the bean.
The Importance of Evaporation
Turning water into steam takes a lot of energy — the energy required is called the الحرارة الكامنة of vaporisation. Understanding this is key to understanding how moisture evaporating affects the way beans respond to heat in the roaster.
If you heat water at a fixed rate, the temperature of the water gradually increases. However, at 100°C, something strange happens. You keep applying heat, but the temperature stops increasing and the water stays at 100°C. That energy is now going towards turning water into steam, instead of increasing the temperature of the water. In fact, it takes five times as much energy to turn water into steam as it does to heat water from 0 to 100°C.
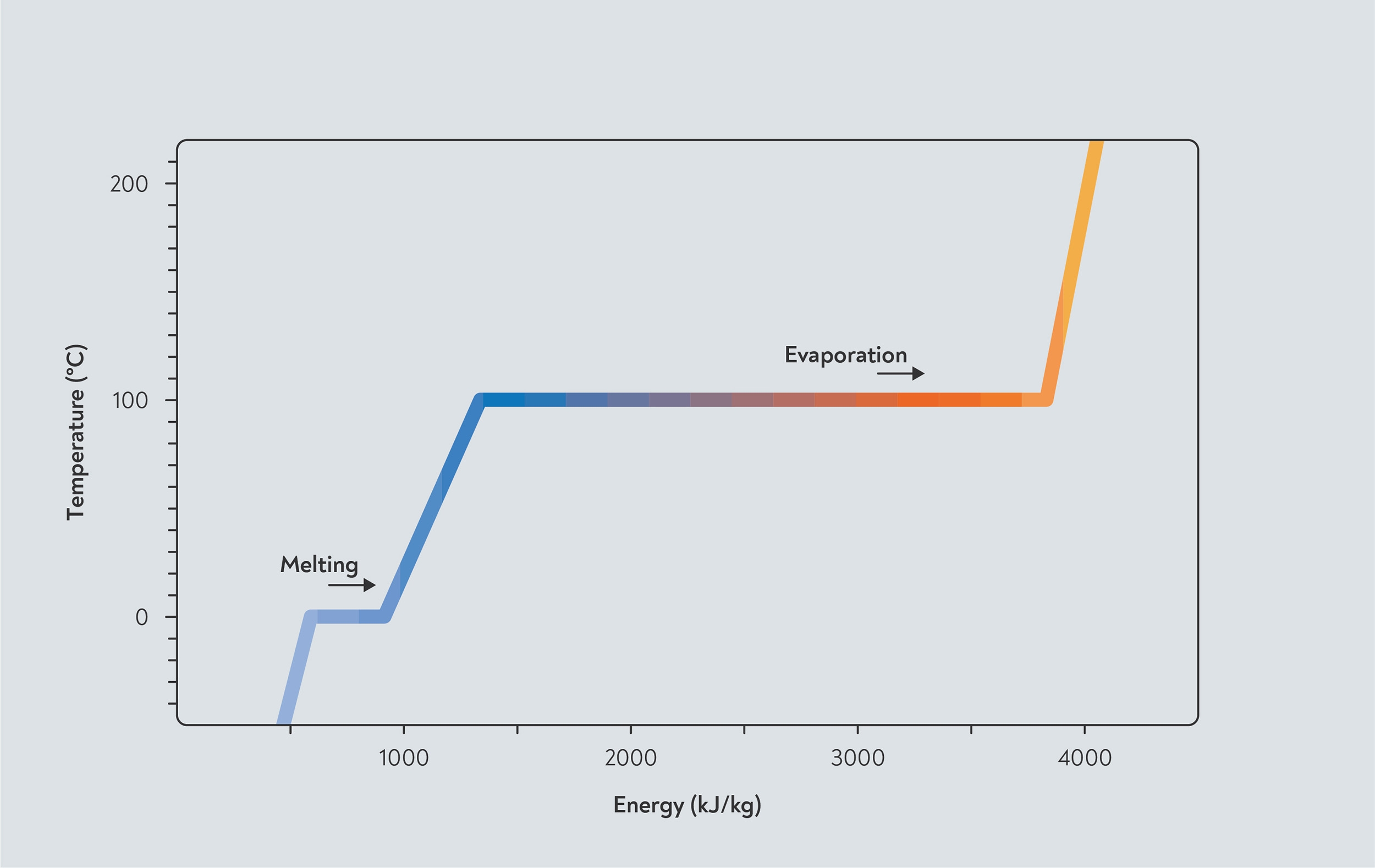 ال الحرارة الكامنة of vaporisation of water. As you add heat, the temperature of water increases up to 100°C but then stops. Any further heat added is used for evaporation, until all the water has turned to steam.
ال الحرارة الكامنة of vaporisation of water. As you add heat, the temperature of water increases up to 100°C but then stops. Any further heat added is used for evaporation, until all the water has turned to steam.
This means that at the evaporation front, a lot of the energy absorbed by the bean is going into vaporising the water, instead of into increasing the temperature. In the outer layer of the bean, on the other hand, that heat can all go into driving chemical reactions and increasing the temperature of the bean.
The effect of this is that any heat that penetrates the bean is able to increase the temperature of the outer layer faster than the inner layer.
Barriers to Heat
As the roast progresses, the outer layer also starts to act as a barrier, reducing the amount of heat reaching the centre of the bean. Firstly, the outer layer dries out. Dry coffee conducts heat less well than moist coffee, so the dry outer layer acts as an additional barrier to heat reaching the centre. Secondly, the outer layer starts to become more porous as the cell structure breaks down. The pores are insulating, because they are filled with gas — just think of how expanded polystyrene works. The result is that the increased volume of pores make the outer layer even less effective at conducting heat to the centre.
Since the outer, dry, layer is less good at conducting heat into the bean, a temperature gradient starts to build up, with the bean surface being hottest. The core of the bean, where the structure is still moist and dense, is more effective at conducting heat. Any heat that reaches this zone is transferred to the core more easily, so the temperature within the core of the bean is more متجانسة.
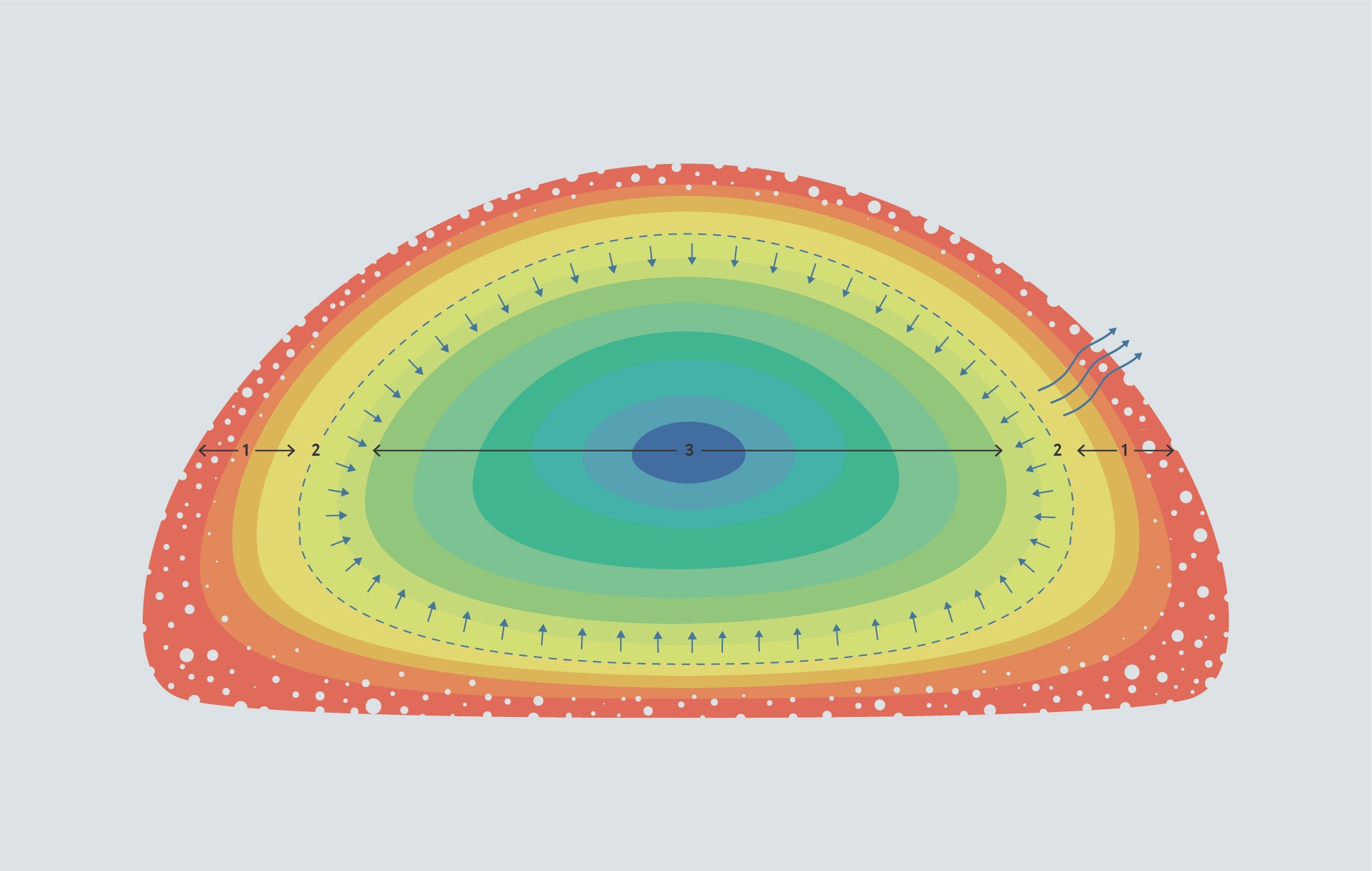 The evaporation front prevents heat from reaching the centre of the bean. In the outer layer of the bean (1), the bean structure is expanded and porous and the moisture content is low, so it doesn’t conduct heat effectively to the inner layers. This results in a steep temperature gradient between the outside and the inside of the bean. At the evaporation front (2), moisture is absorbing heat and converting into steam. The centre of the bean (3), which is denser and has a higher moisture content than the surface, conducts heat more effectively than the surface, so the centre has a more متجانسة temperature
The evaporation front prevents heat from reaching the centre of the bean. In the outer layer of the bean (1), the bean structure is expanded and porous and the moisture content is low, so it doesn’t conduct heat effectively to the inner layers. This results in a steep temperature gradient between the outside and the inside of the bean. At the evaporation front (2), moisture is absorbing heat and converting into steam. The centre of the bean (3), which is denser and has a higher moisture content than the surface, conducts heat more effectively than the surface, so the centre has a more متجانسة temperature
Modelling the Evaporation Front
We can’t see the evaporation front directly — we only surmise it is there because this is how drying behaves in other materials. However, we can predict what happens by using mathematical modelling. In a series of papers, researchers at the University of Oxford and Jacobs Douwe Egberts built a mathematical model to describe the behaviour of moisture inside the bean during roasting.
They found that their model predicted two very distinct zones inside the bean: a high-pressure zone in the centre, continuing both steam and liquid or bound water, and a distinct zone at the edge of the bean that was completely dry — with a narrow evaporation front between them (Fadai et al 2016).
 The evaporation front, as predicted by modelling. Region i contains steam and liquid/bound water at high pressure, and gradually shrinks as the evaporation front moves inwards. Region ii is completely dry. Adapted from Fadai et al (2016)
The evaporation front, as predicted by modelling. Region i contains steam and liquid/bound water at high pressure, and gradually shrinks as the evaporation front moves inwards. Region ii is completely dry. Adapted from Fadai et al (2016)
Surprisingly, their model suggests that the temperature is nearly the same throughout the whole bean. This could imply that the effect of the evaporation front on heat transfer is relatively small, and that the way that moisture escapes from the bean is more important to determining how a coffee roasts than how easily heat penetrates to the centre.
The temperature difference between the centre and the surface of the bean perhaps depends on the roasting conditions. Schenker (2000) inserted a tiny temperature probe into a bean in order to measure the difference between the temperature inside the bean and the temperature of the bean pile, as given by the ‘bean temperature’ probe. How closely the temperature of the core and the bean pile aligned depended on the overall roast speed — faster, hotter roasts showed a bigger difference while in the slower roast, the bean core temperature caught up with the bean pile temperature around half-way through the roast.
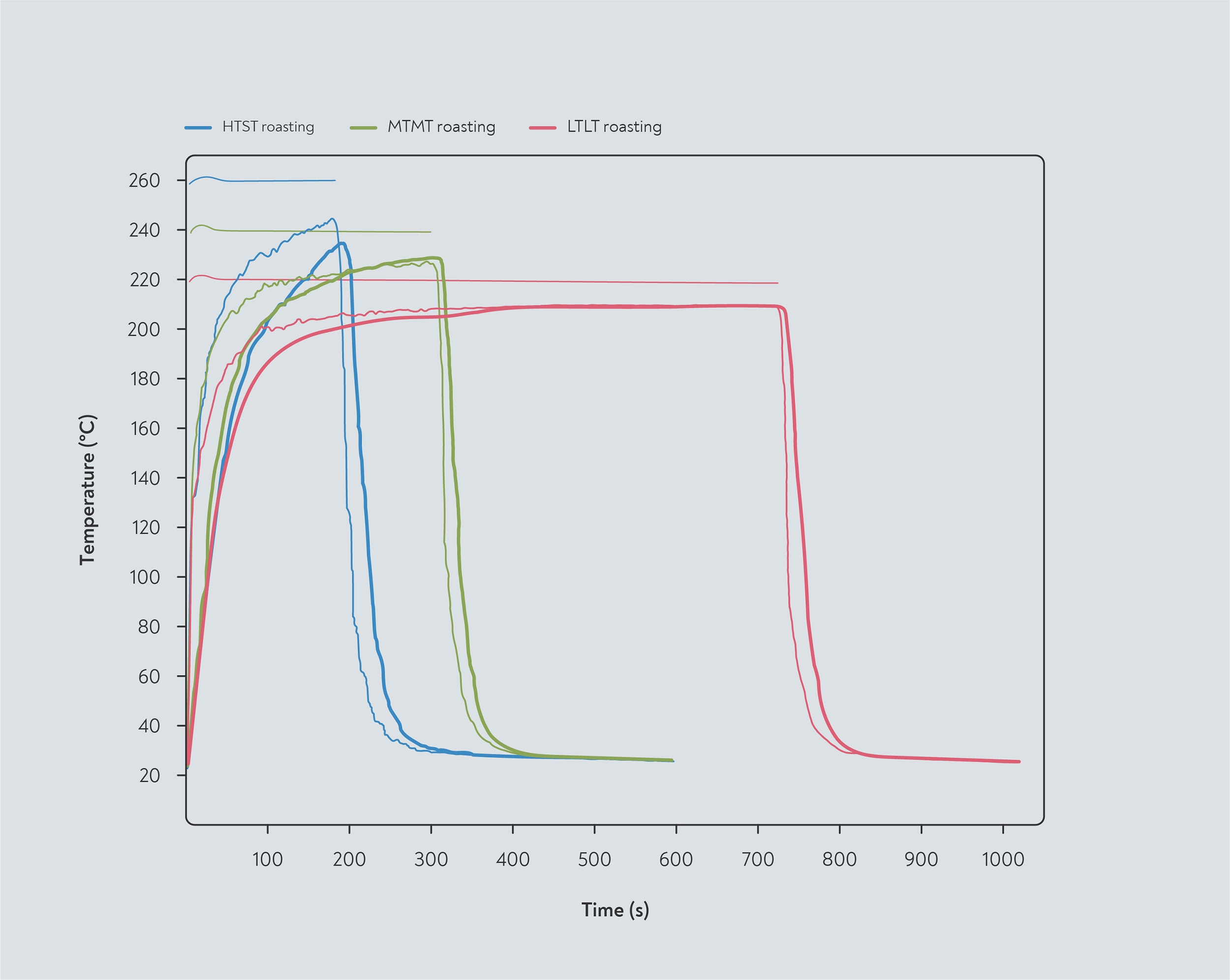 Temperature of the bean pile and bean core during roasting in a fluid-bed roaster operating at fixed temperatures. The bean core temperature lags behind the bean pile but eventually catches up in the slower roast. Three profiles were used: high temperature, short time (HTST); medium temperature, medium time (MTMT); and low temperature, long time (LTLT). Adapted from Schenker (2000)
Temperature of the bean pile and bean core during roasting in a fluid-bed roaster operating at fixed temperatures. The bean core temperature lags behind the bean pile but eventually catches up in the slower roast. Three profiles were used: high temperature, short time (HTST); medium temperature, medium time (MTMT); and low temperature, long time (LTLT). Adapted from Schenker (2000)
The Evaporation Front and First Crack
The evaporation front has one other important effect. As the temperature of the bean increases, at some point the bean material undergoes a transition from a rubbery state to a هشًا one — called the glass transition. The temperature this happens at depends on the amount of moisture — if there’s more moisture, the glass transition happens at a lower temperature.
Incorporating this into the model of the evaporation front has an interesting effect: at a certain temperature, the core of the bean undergoes the glass transition as it has more moisture, and becomes rubbery and able to expand with the build up of steam pressure. The outer layer of the bean, on the other hand, remains هشًا because it is dryer, and so resists that expansion. As the bean temperature increases, stress builds up as the inner region tries to swell but is contained by the هشًا outer layer. This stress could be the cause of first crack (Fadai et al 2019).
Getting a better understanding of how moisture escapes from the bean may turn out to explain a lot more about coffee roasting than we previously thought. If the stress created between the inner and outer layers of the beans is the cause of first crack, then modifying how moisture escapes from the bean, or controlling when the glass transition takes place in the different layers of the coffee, could have profound implications for controlling the overall roast profile.




0 تعليق